Abstract
INTRODUCTION: With the evolving CML treatment landscape, improvement in quality of life and treatment-free remission have become important therapy goals in CML-CP. In the 1st line (1L), more pts are able to achieve DMRs with 2nd-generation (2G) tyrosine kinase inhibitors (TKIs) than IMA. However, 30% to 60% of pts treated with 2G TKIs do not achieve DMR after 5 y of therapy, and 2G TKIs are associated with increased toxicity. Safe and effective treatment options are needed in early lines to help more pts achieve durable DMR.
ASC is the 1st BCR::ABL1 inhibitor to Specifically Target the ABL Myristoyl Pocket (STAMP). ASC was 1st approved in the US for the treatment of adult pts with Philadelphia chromosome-positive CML-CP after ≥2 prior TKIs and pts with the T315I mutation. In the phase 3 ASCEMBL study, ASC led to superior major molecular response vs bosutinib (BOS) at wk 24 and 96. More pts on ASC vs BOS achieved MR4 (wk 24: 10.8% vs 5.3%; wk 48: 10.8% vs 3.9%; wk 96: 17.2% vs 10.5%) and MR4.5 (wk 24: 8.9% vs 1.3%; wk 48: 7.6% vs 1.3%; wk 96: 10.8% vs 5.3%). ASC demonstrated a favorable safety profile. Here, we present results from the ASC4MORE study (NCT03578367) of ASC add-on to IMA vs continued IMA vs switch to NIL in pts not achieving DMR with ≥1 y of 1L IMA (cutoff: Jan 10, 2022).
METHODS: In this open-label, multicenter, qualitative trial, eligible adults with CML-CP were randomized 1:1:1:1 to receive ASC (40 or 60 mg once daily [QD]) add-on to IMA 400 mg QD, continue IMA 400 mg QD, or switch to NIL 300 mg twice daily. The primary endpoint was MR4.5 rate at wk 48. Pts in the IMA arm could switch to the ASC 60-mg QD add-on arm if they had not achieved MR4.5 at wk 48; data from these pts will be analyzed separately and are not part of the current analysis. Pts must have received 1L IMA 400 mg QD for ≥1 y with BCR::ABL1IS >0.01% to ≤1% at randomization. Pts were excluded if they met failure criteria (per European LeukemiaNet 2013), had previous treatment with any TKIs other than IMA, or had achieved DMR at any time with IMA.
RESULTS: Eighty-four pts were randomized to receive ASC 40- or 60-mg QD add-on to IMA, continue IMA, or switch to NIL (n=21 each); baseline characteristics were mostly well-balanced between arms. At cutoff, 85.7%, 76.2%, 23.8%, and 61.9% of pts, respectively, remained on treatment, with median (range) durations of exposure of 104.7 (27-160), 94.0 (1-148), 78.9 (1-146), and 53.4 (50-142) wk, respectively. Top reasons for discontinuation were pt decision in the ASC 40-mg add-on arm (9.5%), AEs in the ASC 60-mg add-on and NIL arms (14.3% and 23.8%, respectively), and physician decision in the IMA arm (57.1%) (pts who crossed over to the ASC 60-mg add-on arm were considered discontinued for IMA arm).
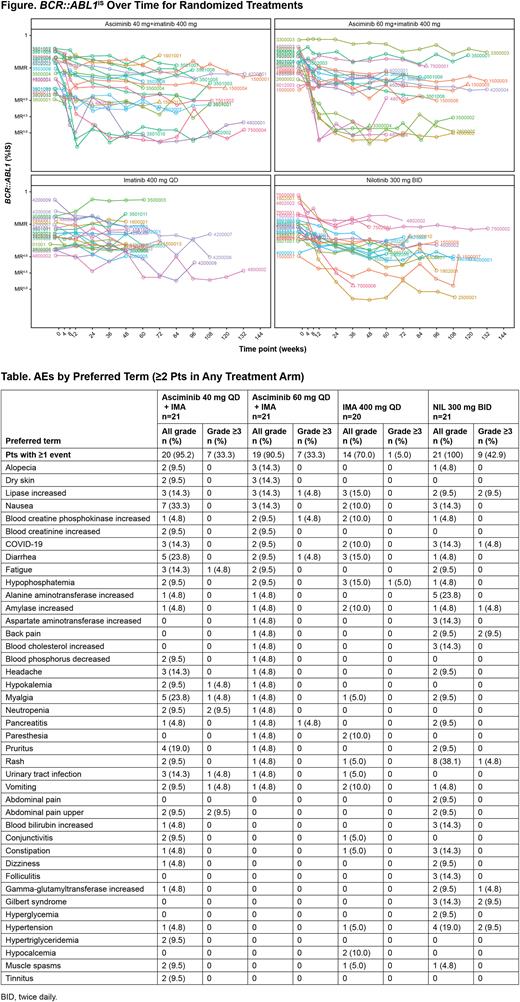
In the 40-mg ASC add-on, 60-mg ASC add-on, IMA, and NIL arms, respectively, 19.0%, 28.6%, 0%, and 4.8% of pts were in MR4.5 at wk 48, with 42.9%, 28.6%, 0%, and 23.8% in MR4 at wk 48. DMRs were achieved earlier in pts receiving ASC 40- and 60-mg add-on vs NIL; median time to MR4.5 was 12.1, 12.4, and 24.6 wk, respectively (Figure). The Kaplan-Meier-estimated rate of maintaining MR4.5 for ≥48 wk was 60.0%, 80.0%, and 66.7% in the 40- and 60-mg ASC add-on and NIL arms, respectively.
As expected in these pts tolerating IMA for ≥1 y, ASC add-on was associated with higher rates of adverse events (AEs), serious AEs, and discontinuations than continued IMA, but less than switching to NIL. AEs leading to discontinuation in the 40-mg add-on, 60-mg ASC add-on, IMA, and NIL arms were reported in 4.8%, 14.3%, 0%, and 23.8% of pts, respectively, with dose adjustments/interruptions occurring in 28.6%, 33.3%, 5.0%, and 23.8%, respectively. There were no new or worsening safety findings with ASC (Table).
CONCLUSIONS: More pts achieved DMR at wk 48 with ASC add-on to IMA vs continued IMA or switch to NIL in pts not achieving DMR with IMA alone for ≥1 y. In this population of pts tolerating IMA for ≥1 y, ASC add-on was generally well tolerated. While not powered to identify the best arm for achievement of DMR in this setting, the high rate of DMR in the ASC add-on arms is promising. Further studies are needed to assess if ASC alone can provide equivalent efficacy and better tolerability vs add-on to IMA.
Ongoing studies are assessing ASC monotherapy in 1L, including ASC4FIRST (NCT04971226) and ASC4START. ASC may provide an important early-line treatment option to help more pts safely achieve rapid and deep responses and reduce therapy switching.
Disclosures
Cortes:Bristol Myers Squibb: Consultancy, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Forma Therapuetic: Consultancy; Sun Pharma: Consultancy, Research Funding; Biopath Holdings: Consultancy, Current equity holder in private company; Gilead: Consultancy; Pfizer: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Research Funding; Kartos: Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Hughes:Novartis: Consultancy, Research Funding; Enliven: Consultancy, Research Funding; BMS: Consultancy, Research Funding. Kim:Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Otsuka: Consultancy, Honoraria, Research Funding, Speakers Bureau; Il-Yang: Consultancy, Honoraria, Research Funding, Speakers Bureau. Lomaia:Fusion Pharma: Speakers Bureau; Bristol Myers Squibb: Other: Travel, Accommodation, Expenses ; Pfizer: Other: Travel, Accommodation, Expenses , Speakers Bureau; Novartis: Other: Travel, Accommodation, Expenses , Speakers Bureau. Mayer:Novartis: Other: Travel support, Research Funding. Turkina:Fusion Pharma: Speakers Bureau; Pfizer: Other: travel, accommodation expenses, Speakers Bureau; Novartis: Other: travel, accommodation expenses, Speakers Bureau. Hurwicz Kogut:Novartis: Consultancy. Torres Cardoso:Novartis: Current Employment. Sura:Novartis: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal